Intuitive Surgical Revolutionizes Lung Biopsies with AI
Intuitive Surgical's FDA-cleared AI and imaging upgrades enhance lung biopsy precision, advancing early cancer diagnosis and treatment.
Intuitive Surgical Revolutionizes Lung Biopsies with AI
Intuitive Surgical, a leader in robotic-assisted surgery, announced on October 8, 2025, that the U.S. Food and Drug Administration (FDA) has cleared significant software upgrades for its Ion Endoluminal System. This update integrates advanced artificial intelligence (AI) navigation and enhanced imaging capabilities designed to improve the precision and efficiency of minimally invasive lung biopsies. The new features address critical clinical challenges such as CT-to-body divergence—a phenomenon where lung nodules shift between preoperative imaging and the actual procedure—thus advancing early lung cancer diagnosis and treatment.
Background: The Ion Endoluminal System and Lung Cancer Diagnostics
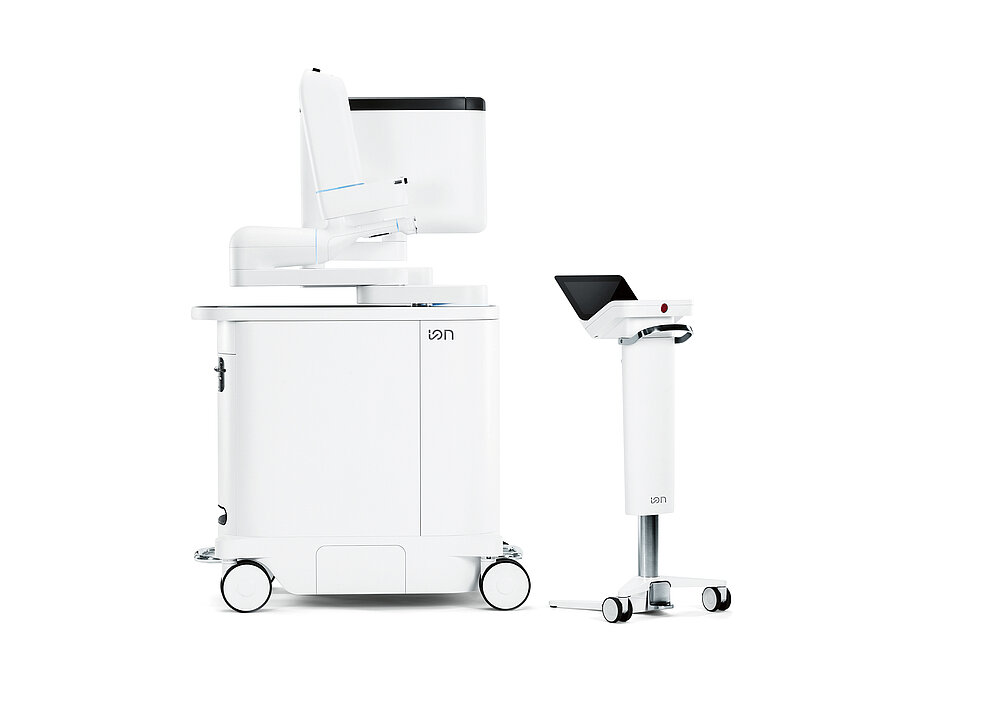
The Ion Endoluminal System is a robotic-assisted bronchoscopy platform that uses an ultra-thin, shape-sensing catheter to navigate deep into the lungs. This allows physicians to access small or hard-to-reach lung nodules for biopsy with greater precision than traditional methods. Lung cancer remains the leading cause of cancer-related deaths globally, with early and accurate diagnosis being essential to improving survival rates. Ion's minimally invasive approach supports this goal by enabling targeted biopsies that reduce patient risk and enhance diagnostic accuracy.
As of mid-2025, more than 900 Ion systems have been installed across 10 countries, supporting clinical research involving over 2,000 subjects. This broad adoption reflects growing clinician confidence and the system's potential impact on lung cancer care.
Key Features of the FDA-Cleared Software Update
AI-Driven Navigation to Address CT-to-Body Divergence
A core advancement is the integration of AI-powered navigation designed to detect and correct CT-to-body divergence in real time. This issue arises when lung nodules shift position between the preoperative CT scan and the actual biopsy procedure due to changes in lung volume or patient positioning. By combining computer vision with Ion’s shape-sensing catheter technology, the system recalibrates the navigation pathway dynamically, similar to how GPS reroutes when a driver deviates from a planned route. This innovation is expected to:
- Enhance biopsy targeting accuracy
- Reduce the need for manual navigational adjustments
- Streamline the procedural workflow, potentially shortening procedure times
Advanced Imaging Integration Including Tomosynthesis
The update also introduces expanded imaging capabilities with integrated tomosynthesis, a form of 3D imaging that utilizes standard 2D C-arm equipment commonly available in hospitals. This allows real-time imaging updates during the biopsy procedure, even in settings lacking full 3D imaging resources. The imaging enhancements enable clinicians to:
- Obtain real-time visualization of lung anatomy and biopsy tools
- Tailor imaging workflows to individual patient needs
- Expand access to advanced imaging in diverse clinical environments, including community hospitals
Industry and Market Impact
The FDA clearance of these AI and imaging upgrades solidifies Intuitive's leadership in the robotic-assisted lung biopsy market. By enhancing Ion’s clinical utility and procedural precision, the company is positioned to capture a larger share of the growing minimally invasive diagnostics sector, which is critical as lung cancer screening and early detection initiatives expand worldwide.
Following the announcement, Intuitive’s stock (NASDAQ: ISRG) rose 1.6%, reflecting investor confidence in the product’s commercial potential. Despite a challenging year-to-date performance relative to the broader market, this upgrade is expected to boost adoption and recurring instrument usage, driving new revenue streams.
Future Outlook and Deployment Plans
Intuitive plans a limited U.S. launch of the upgraded Ion system immediately, with a broader rollout scheduled for 2026. The company will continue to support clinical research and real-world evidence gathering to validate the benefits of AI-enhanced navigation and advanced imaging in improving lung biopsy outcomes.
With the integration of AI and advanced imaging, Intuitive is not only improving procedural accuracy but also reinforcing its ecosystem advantage in early-stage cancer care. This development aligns with the company’s mission to drive minimally invasive innovation that improves patient outcomes globally.
Relevant Visuals for This Story
- Ion Endoluminal System: Official product images showing the robotic bronchoscopy platform and shape-sensing catheter technology.
- Intuitive Surgical Logo: Corporate branding reflecting the company’s identity and leadership.
- Illustrations of AI Navigation: Visual representations of AI correcting CT-to-body divergence and real-time imaging during lung biopsy.
- Tomosynthesis Imaging Examples: Images demonstrating the use of tomosynthesis with C-arm equipment for lung nodule visualization.
These visuals help contextualize the technology’s impact and the sophistication of the integrated AI and imaging systems.
Intuitive Surgical’s newest FDA-cleared software update for the Ion Endoluminal System marks a pivotal advancement in lung cancer diagnostics, leveraging AI and imaging innovations to enhance biopsy accuracy, procedural efficiency, and accessibility across clinical settings—an important stride toward improving survival rates in lung cancer patients worldwide.



